Talks by our group members

Tom - Synthetic Biology
Studying natural systems to create functional synthetic mole
Antti - DNA26
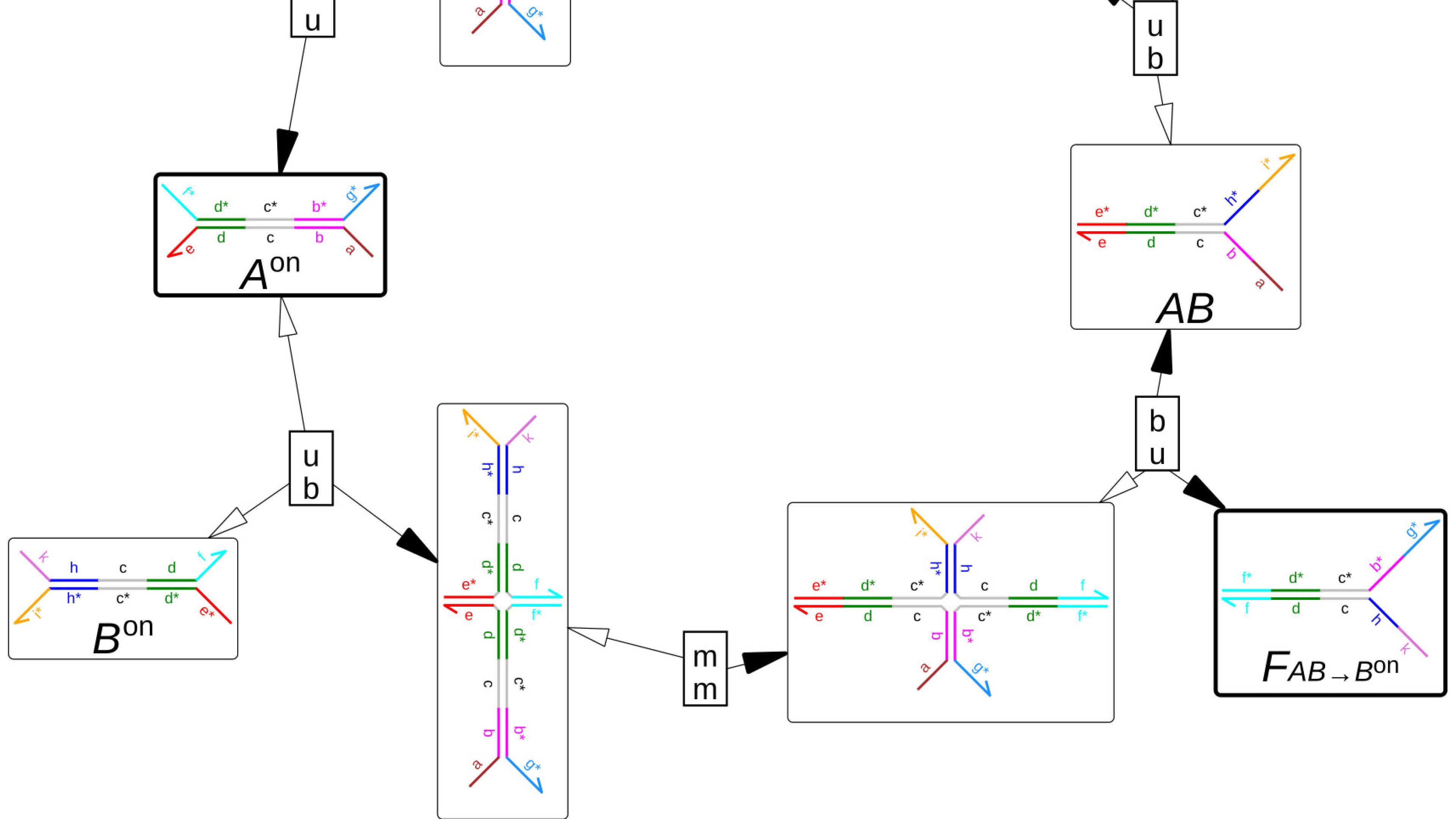
Implementing Non-Equilibrium Networks with Active Circuits of Duplex Catalysts
Implementing Non-Equilibrium Networks with Active Circuits of Duplex Catalysts
DNA strand displacement (DSD) reactions have been used to construct chemical reaction networks in which species act catalytically at the level of the overall stoichiometry of reactions. These effective catalytic reactions are typically realised through one or more of the following: many-stranded gate complexes to coordinate the catalysis, indirect interaction between the catalyst and its substrate, and the recovery of a distinct "catalyst" strand from the one that triggered the reaction. These facts make emulation of the out-of-equilibrium catalytic circuitry of living cells more difficult. Here, we propose a new framework for constructing catalytic DSD networks: Active Circuits of Duplex Catalysts (ACDC). ACDC components are all double-stranded complexes, with reactions occurring through 4-way strand exchange. Catalysts directly bind to their substrates, and the "identity" strand of the catalyst recovered at the end of a reaction is the same molecule as the one that initiated it. We analyse the capability of the framework to implement catalytic circuits analogous to phosphorylation networks in living cells. We also propose two methods of systematically introducing mismatches within DNA strands to avoid leak reactions and introduce driving through net base pair formation. We then combine these results into a compiler to automate the process of designing DNA strands that realise any catalytic network allowed by our framework.
https://drops.dagstuhl.de/opus/volltexte/2020/12960/pdf/LIPIcs-DNA-2020-7.pdf

Tom - WOST III
Avoiding equilibrium in a minimal molecular information-processing system
Avoiding equilibrium in a minimal molecular information-processing system
Tom - Banff 2020
Non-Equilibrium Thermodynamics of Catalytic Information Processing
Non-Equilibrium Thermodynamics of Catalytic Information Processing
Tom's talk at the Banff International Research Station workshop "Mathematical Models in Biology: from Information Theory to Thermodynamics" (2020).
Catalytic motifs are ubiquitous in cellular information-processing systems, from kinase signalling networks to the central dogma of molecular biology. This ubiquity results from the ability of catalysts to channel chemical free energy into far-from-equilibrium information-bearing states, allowing them to perform non-trivial computational operations. This power, however, comes at a price. At a fundamental level, the need to create non-equilibrium outputs sets thermodynamic constraints on these systems. At a practical level, catalysts must carefully balance kinetic and thermodynamic factors to ensure that the desired non-equilibrium output is actually reached. The complexity of this task explains the comparatively slow progress made with engineering synthetic non-equilibrium information-processing systems, as opposed to synthetic systems that form complex equilibrium assemblies. I will present our latest work - both theoretical and experimental - aimed at overcoming this challenge to engineer non-equilibrium catalytic systems for information processing.
Papers from which this talk is drawn:
https://link.springer.com/article/10.1007/s11047-017-9646-x
Javi - FNANO2020
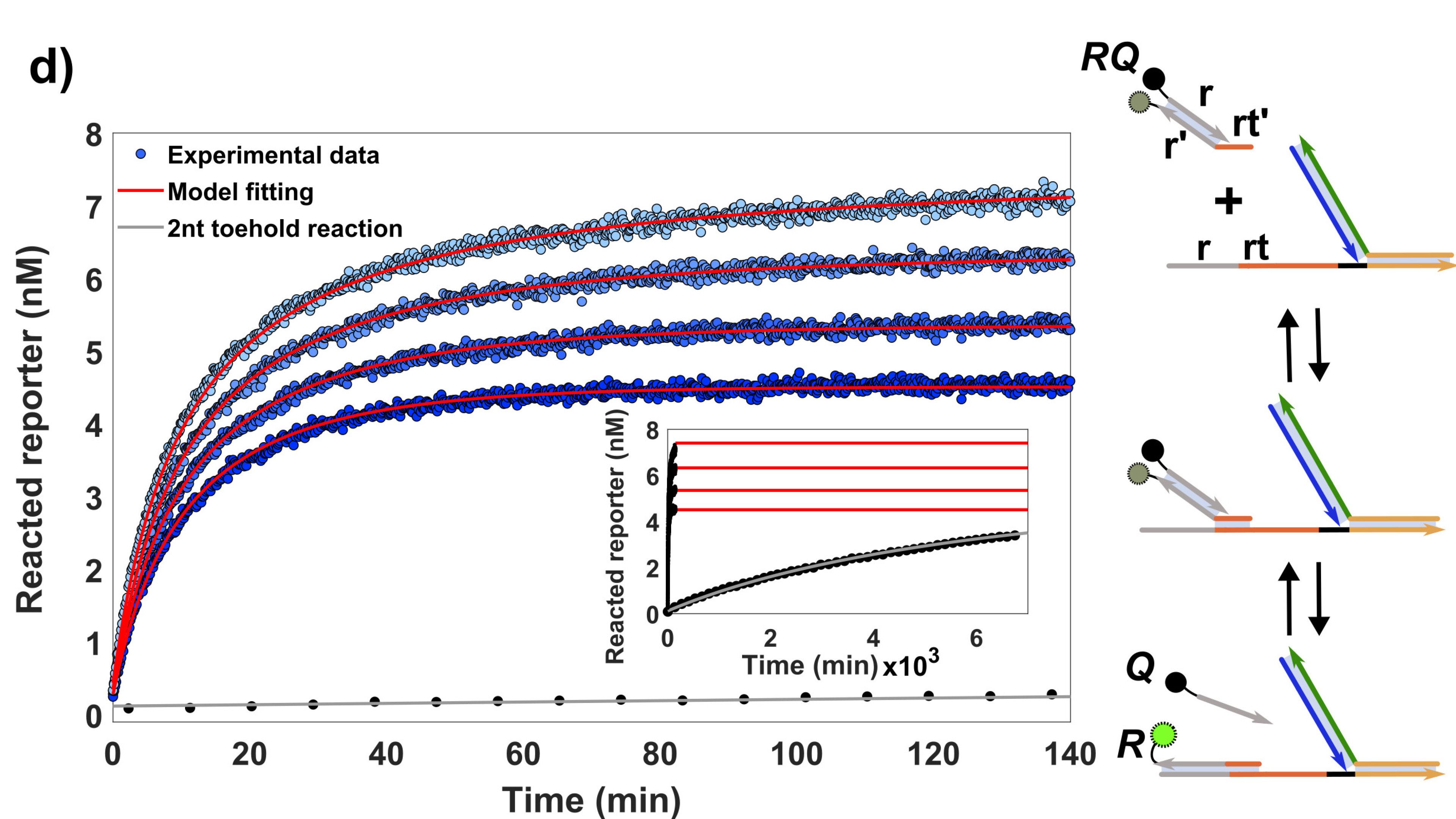
Handhold-mediated strand displacement for non-equilibrium templating
Handhold-mediated strand displacement for non-equilibrium templating
Toehold-mediated strand displacement (TMSD) is a nucleic acid-based reaction wherein an invader strand (I) replaces an incumbent strand (N) in a duplex with a target strand (T). TMSD is driven by toeholds, overhanging single-stranded domains in T recognised by I. Although TMSD is powerful, it cannot implement templating, the mechanism by which biological systems generate far-from equilibrium assemblies like RNA or proteins. Therefore, we introduce handhold-mediated strand displacement (HMSD). Handholds are toehold analogues located in N and capable of implementing templating. We demonstrate that handholds can accelerate the rate of invader-target (IT) binding by more than 4 orders of magnitude. Furthermore, handholds of moderate length accelerate reactions whilst allowing detachment of the product IT from N. We are thus able to experimentally demonstrate the use of HMSD-based templating to produce highly-specific far-from-equilibrium DNA duplexes.

Javi - three minute thesis
The Copy Dilemna
A broad intro to Javi's work for Imperial's three-minute-thesis competition.